Contributions to the Periodic Table
Category: Иное
Обновлено: 20 фев 2023
Авторы
Created byAnh Phan
Attachments
События
Antoine Lavoisier:
He discovered four elements
which were hydrogen, oxygen,
carbon and silicon. Moreover,
he formed the law of conservation
of mass which states that in a chemical
change, no atoms are created nor destroyed
along with the establishment of chemical
nomenclature which is a naming system
for chemical compounds.
Additionally, Lavoisier contributed to the
periodic table by being the first person
to classify elements into metals and
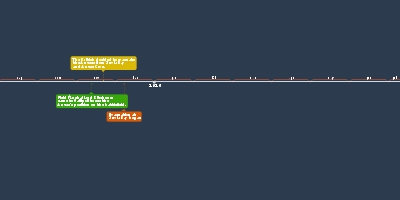
non-metals.Johann Wolfang Dobereiner:
Dobereiner is perhaps most well-known for
inventing the first commercial lighter which
was called a 'tinderbox' or the Dobereiner lamp.
It was used to light fires and pipes for smoking.
Besides that, he also made some contributions
to the periodic table. He split
those elements into triads which are groups of
three and arranged those elements
in ascending order of atomic weights.
Due to this, he saw that the
middle element had properties that were
the average of the other two.John Newlands:
Newlands was the first person to arrange each
of the elements known at the time, periodically
in the order of atomic mass. This arrangement
allowed him to see that every eight elements
had similarities which he called the law of octaves.
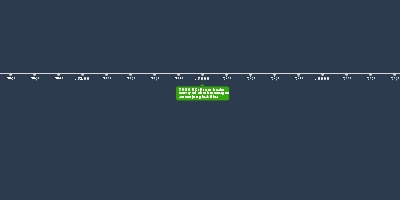
No gaps were left for new elements.Dmitri Mendeleev:
Mendeleev, a polymath created the modern structure of the periodic table.
The elements were arranged in order of atomic weight and into various groups
based on their compositions. One creative idea that is unique in Mendeleev's
contribution to the periodic table was how he left gaps for future elements such
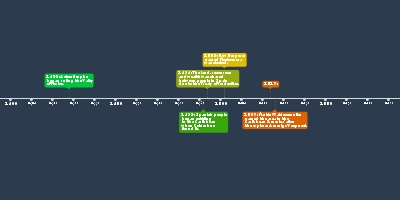
as gallium and was able to predict their properties.Lothar Meyer:
Meyer arranged the elements into a periodic
table that was quite similar to Mendeleev's and
he also left gaps for some future elements.
However, he was never able to predict
their properties. One creative idea was his
discovery of the Periodic Law.Henry Moseley:
Moseley played a big part in the modern periodic table.
He had a creative idea which was to use the
concept of subatomic particles to group the
elements. In his experiments, Moseley used
X-ray to find out the approximate wavelengths
of each element and gave them each a
correlated atomic number. Later on,
he regrouped these elements into a new
periodic table in order of their atomic number
and into groups which helped to clear some
inconsistencies found in previous tables.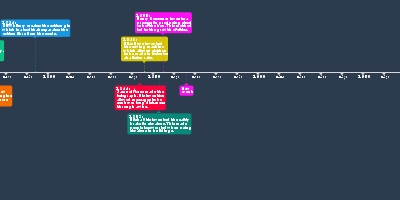
Comments