feb 14, 2018 - James Clark
Description:
Summary:
The problem with Chadwick's model is that electrons have no evidence that they orbit the nucleus like those of planets around the sun. However, that is about as close as a physical model can actually get us because we, as humans are relying on the laws of quantum mechanics to work on electrons just as everything else that we know of does. For instance, the principle of accuracy states that, through knowing the location of an electron at a particular time, we are no closer to finding out anything about its movements. Therefore, as most chemists do these days, we consider instead using mathematics to find out things about electrons. These things include its speed, energy, spin and even mass. WHat results that have been found to result in something that looks a little like the 3rd picture down. However, as I am not equipped to figure out the rest of these important figures, I cannot make my own theory of the atom, other than the fact that it has a dense nucleus, filled with protons and neutrons.
References:
-Atomic Theory - body, used, process, law, chemical, form, energy, parts, effects. (2018). Scienceclarified.com. Retrieved 12 February 2018, from http://www.scienceclarified.com/As-Bi/Atomic-Theory.html
-Lucarelli, N. (2014). Essential chemistry (pp. 11-17). Willetton, W.A.: Lucas Publications.
Pictures:
Top: Portrait
Middle: Development of the atom
Bottom: Probability of what the positioning and movement of an electron in the atom is.
Added to timeline:
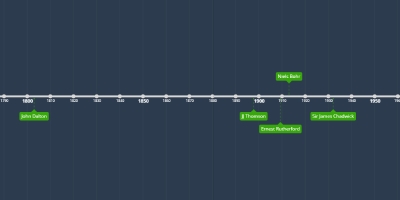
History of the atom
Date:
Images:
![]()
![]()
![]()
