Chemical Revolution
Category: Other
Updated: 6 Nov 2018
Chemical Revolution Timeline
Contributors

Created byJohn LD
Attachments
Events
Joseph Black isolates 'fixed air' (carbon dioxide). Upon heating, calcium carbonate (CaCO3) produced a gas that was denser than air and could not sustain fire or animal life.Henry Cavendish produces 'inflammable air' (hydrogen) by bringing acids into contact with metals, forming water on combustion.Priestley isolates 'phlogisticated nitrous air'.Priestley's candle / mint / bird dome experiments.Sunlight focused on mercury to produce 'dephlogisticated air' which is 'five or six times better than common air.'Discovery that a blood clot will turn red when exposed to dephlogisticated air, and black when exposed to airs rich in phlogiston.Priestley and Lavoisier meet in Paris and discuss Priestley's latest findings.Marie Paulze marries Lavoisier.Lavoisier elected to Royal Academy of ScienceLavoisier reads Guyton de Morveau's on the weight of phlogiston.Priestley's "Directions for Impregnating Water with Fixed Air" is publishedLavoisier deposits his sealed memoir.Lavoisier succeeds in measuring the weight gained by phosphorous during combustion.Discovers that 'pure air' is the source of acidity in nitrous acid. Declares that 'pure air' makes up 25% of the atmosphere.Lavoisier's first use of the term 'oxygen'.Produced water by heating lead oxide in an atmosphere of hydrogen.Mercury tube experiment, producing ‘inflammable air’ renamed ‘hydrogen’Periods
Lavoisier's experiments on the weight of phosphorous, and calcination of metals.Returns to the air obtained from calx of mercury. Determines this is not 'fixed air' (CO2). It is 'elemental air.'Experiments on respiration, determination that the blood absorbs pure elemental air.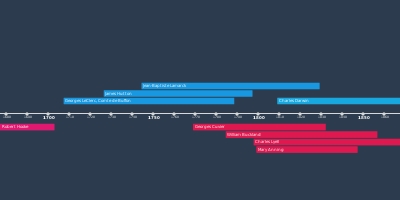
Comments