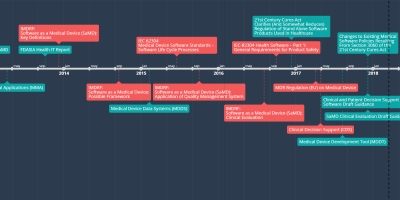
dec 8, 2017 - SaMD Clinical Evaluation Draft Guidance
Description:
The draft guidance pertains to the conduct of clinical evaluation of Software as a Medical Device (SaMD) and focuses on the general principles of clinical evaluation, which includes establishing the scientific validity, clinical performance, and analytical validity for a SaMD. This draft guidance is not final nor is it in effect at this time.Added to timeline:
Date:
dec 8, 2017
Now
~ 6 years and 5 months ago