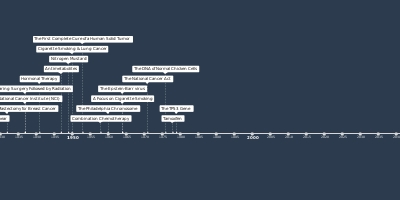
jan 1, 2011 - Ipilimumab
Description:
For the diagnosis of inoperable or metastatic melanoma, the Food and Drug Administration (FDA) permits the use of ipilimumab, a monoclonal antibody. Ipilimumab stimulates the immune system by removing a "brake" that normally controls the intensity of immune responses to attack cancer cells.Added to timeline:
Date:
jan 1, 2011
Now
~ 14 years ago