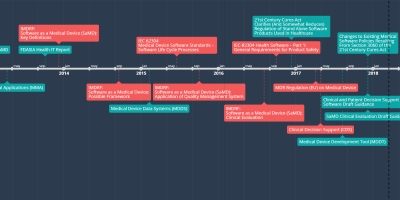
8 dic 2017 año - SaMD Clinical Evaluation Draft Guidance
Descripción:
The draft guidance pertains to the conduct of clinical evaluation of Software as a Medical Device (SaMD) and focuses on the general principles of clinical evaluation, which includes establishing the scientific validity, clinical performance, and analytical validity for a SaMD. This draft guidance is not final nor is it in effect at this time.Añadido al timeline:
fecha:
8 dic 2017 año
Ahora mismo
~ 6 years and 5 months ago