Scientistic discoveries of the Atom
Category: Outro
Atualizado: 7 fev 2020
Autores
Created byMatthew Lockwood
Attachments
Eventos
John Dalton - discovered the solid sphere
model called "atoms" from the Greek word
'atomos'Positive - atoms of 1 element differ from anotherNegative - Atoms aren't divisibleJ J Thomson - "Plum Pudding" model
-Discovered Electrons
-No NucleusPositive - Positive charges in the centreNegative - No NucleusErnst Rutherford - Nuclear Model
-Discovered the Nucleus and the atoms are mostly empty spacePositive - Positive charges in the centerNegative - No explanation for electron orbitsNeils Bohr - Planetary Model
- Electrons orbited the Nucleus in fixed orbits of differing energy levelPositive - Stable electron orbits with emission spectraNegative - Doesn't work for heavier elementsErwin Schrodinger - Quantum Model
- Electrons don't move in fixed orbits but in waves
- Impossible to locate electrons (cloud of possibility)Positive - Most widely accepted accurate model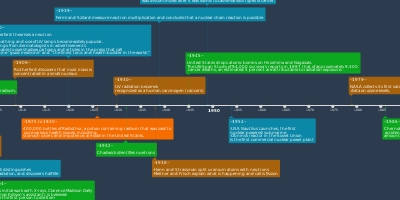
Comments