8 dez 2017 ano - Changes to Existing Medical
Software Policies Resulting
From Section 3060 of the
21st Century Cures Act
Descrição:
A draft guidance that provides clarity on FDA’s current thinking regarding changes made by the 21st Century Cures Act (Cures Act) to the definition of a medical device and the resulting effect on guidances related to medical device software. This draft guidance is not final nor is it in effect at this time.
Adicionado na linha do tempo:
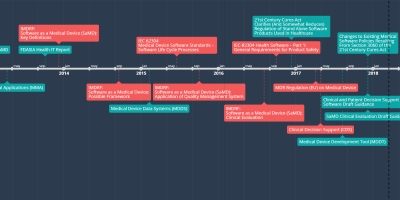
SAMD Timeline
Data:
