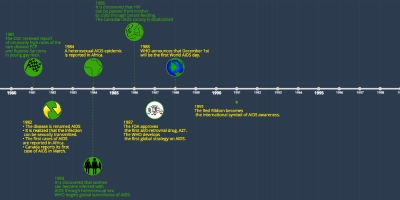
5 jan 2016 ano - 2016 Health Canada approves use of daily oral Truvada (tenofovir plus FTC) for use as pre- exposure prophylaxis (PrEP) to reduce the risk of sexual transmission of HIV. Updated results from the HPTN 052 and PARTNER studies continue to show that antiretroviral treatment (ART) and an undetectable viral load significantly reduces the risk for HIV transmission through both anal and vaginal sex
Adicionado na linha do tempo:
Data:
5 jan 2016 ano
Agora
~ 8 years and 4 months ago