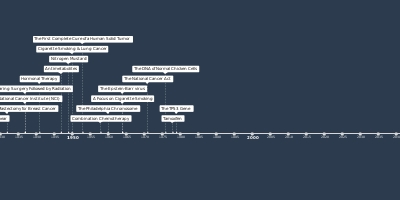
1 janv. 1997 - Rituximab
Description:
For use in patients with treatment-resistant, low-grade or follicular B-cell non-Hodgkin lymphoma (NHL), the Food and Drug Administration (FDA) approves rituximab, a monoclonal antibody.Ajouté au bande de temps:
Date:
1 janv. 1997
Maintenaint
~ Il y a 28 ans