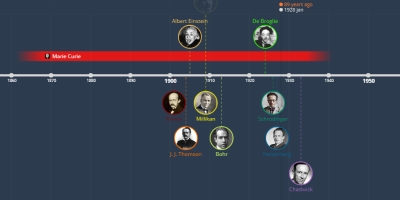
1 janv. 1909 - Rutherford
Description:
Nuclear model of the atom in which: a very small positively charged nucleus containing most of the mass of the atom; a very large volume around the nucleus in which electrons move; a nucleus containing positively charged protons; a number of protons equal to the number of electrons. He later postulated the existence of a neutral particle in the nucleus to make up for the calculated mass deficiency in the atoms studied.Ajouté au bande de temps:
Date:
1 janv. 1909
Maintenaint
~ Il y a 116 ans