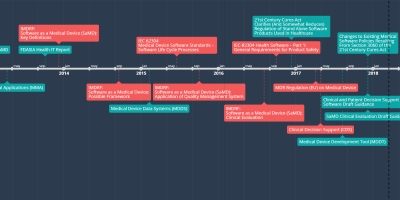
oct 2, 2015 - IMDRF:
Software as a Medical Device (SaMD):
Application of Quality Management System
Description:
This document provides guidance on the application of existing standardized and generally accepted QMS practices to SaMD. This guidance informs the reader of SaMD specific practices, highlight SaMD realization and use processes from the perspective of patient safety and clinical environment considerations, help manufacturers and regulators attain a common understanding and vocabulary for the application of medical device qualify management system requirements to SaMD, and complement the IMDRF SaMD framework for risk categorization.
Added to timeline:
SAMD Timeline
Date:
