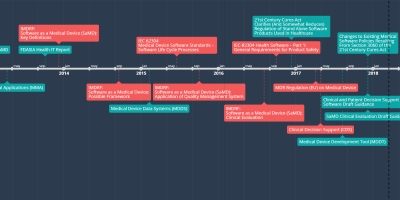
jun 18, 2015 - IEC 62304
Medical Device Software Standards –
Software Life Cycle Processes
Description:
This document defines the life cycle requirements for medical device software. The set of processes, activities, and tasks described in this standard establishes a common framework for medical device software life cycle processes.
Added to timeline:
SAMD Timeline
Date:
