5 agos 2016 año - IMDRF:
Software as a Medical Device (SaMD):
Clinical Evaluation
Descripción:
This document provide guidance on clinical evaluation describing relevant clinical evaluation methods and processes, necessary level of clinical evidence for different categories of SaMD, and SaMd categories where independent review is important and not important.
Añadido al timeline:
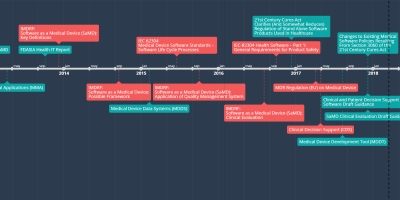
SAMD Timeline
fecha:
